Bone healing is a structured, highly coordinated biological process. Rather than simply ‘closing a gap,’ bone responds to injury through a sequence of events that depend on controlled inflammation, adequate circulation, cellular energy, and the appropriate mechanical stimulus. This becomes especially relevant when managing stress reactions and hairline fractures. Injuries that often present with subtle symptoms but require a precise environment to heal well.
The healing sequence begins with inflammation, which is not only normal but essential. Immune cells clear damaged tissue, activate early repair pathways, and stimulate angiogenesis. As the process transitions out of this phase, the mechanical environment begins to play a larger role. Early stability allows the initial callus to form, and later, progressive loading provides the mechanical signal required for tissue organization and remodeling.
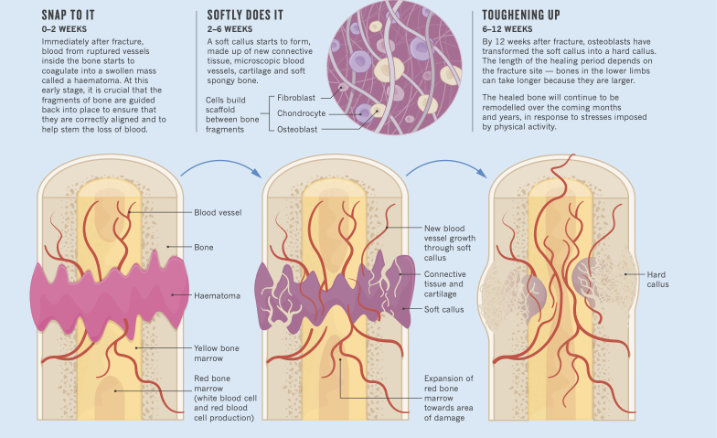
Circulation is a major driver at every stage. Oxygen, minerals, growth factors, and precursor cells reach the injury site through vascular networks. When perfusion is limited, healing slows; when blood flow improves, bone formation is more efficient. On the metabolic side, osteoblasts rely on ATP and stable pH to synthesize new bone. Systemic variables such as stress, sleep, nutrition, endocrine function can either support or compromise this environment.
Within this broader physiological framework, TECAR therapy serves as an adjunct that supports the conditions bone healing depends on. It does not replace loading or directly stimulate osteogenesis but it does improve many of the variables clinicians work to optimize.
TECAR enhances microcirculation and oxygenation, creating a more efficient vascular environment for early repair. It facilitates lymphatic drainage, helping reduce localized congestion without suppressing the inflammatory activity needed for healing. TECAR also increases metabolic activity in deeper tissues, improving ATP availability and overall cellular turnover factors that indirectly support osteoblast function during callus formation and remodeling.
Clinically, TECAR provides another meaningful advantage: it reduces pain without adding mechanical stress. Patients can maintain movement patterns more naturally, avoid compensatory strategies, and begin low-load progressions earlier and more comfortably.
Because TECAR supports circulation, metabolic function, and soft-tissue mobility without stressing the fracture site, it integrates well across the full healing timeline. Early on, it can help manage swelling and promote fluid dynamics. As healing progresses, moderate intensities enhance vascular and metabolic support. In the later phase, TECAR assists with mobility restoration and preparing surrounding tissues for progressive loading.
In short, while TECAR doesn’t heal bone directly, it helps create the environment bone needs to heal well. For clinicians managing bone stress injuries, it offers a targeted way to influence circulation, inflammation, and tissue quality which are key components in guiding patients safely and effectively through recovery.
References
Claes, L., Recknagel, S., & Ignatius, A. (2023). The role of macrophages in fracture healing. Annals of Translational Medicine.
Frontiers in Bioengineering and Biotechnology. (2025). Extended view on the mechanobiology of fracture healing. Frontiers in Bioengineering and Biotechnology.
Gubin, A. V., et al. (2024). Methods to accelerate fracture healing: A narrative review. Journal of Orthopaedic Research.
Hosseini, M., & collaborators. (2025). Repair mechanisms of bone system tissues based on growth factors and gene expression in fracture healing. Cell and Tissue Research.
Iaquinta, M. R., et al. (2025). Bone regeneration: A review of current treatment strategies. Journal of Clinical Medicine, 14(6), 1838.
Vahdatpour, B., et al. (2022). Transfer of energy capacitive and resistive (TECAR) therapy: Clinical effects on musculoskeletal pain and inflammation. Journal of Pain Research, 15, 483–495.
Jang, Y., Lee, G., Lee, S., Na, D., Shin, H., Choi, J. B., & Koh, J. C. (2023). Efficacy of transcutaneous 4.4 MHz radiofrequency diathermy versus therapeutic ultrasound in knee osteoarthritis. Journal of Clinical Medicine, 12(18), 6040.
Szabo, D. A., et al. (2022). Effects of capacitive–resistive radiofrequency therapy on blood flow and deep tissue temperature. Medical Science Monitor, 28, e938873.
Lupowitz, L. G., Ramus, L., Delacour, F., & Johnson, K. (2025). Capacitive-resistive monopolar radiofrequency therapy (CRMRF): Metabolic effects and clinical applications. International Journal of Sports Physical Therapy.

